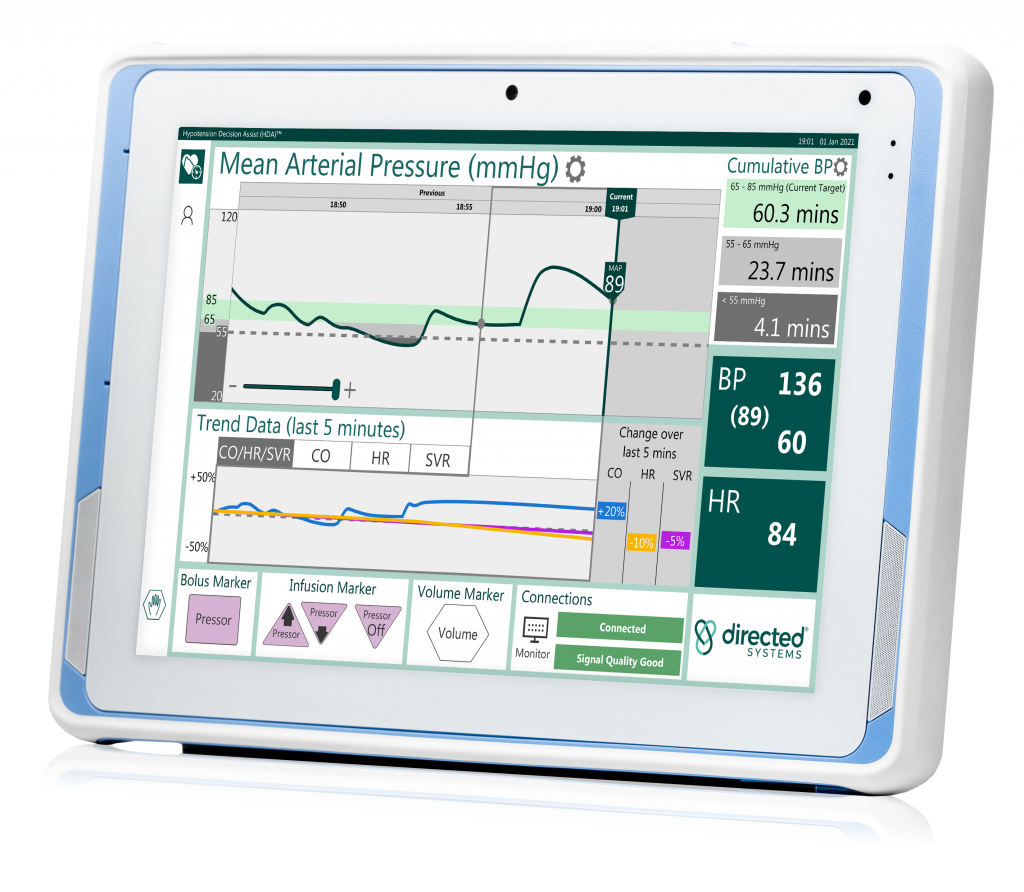
Directed systems Ltd anounces a brand-new version of, and fDA 510(k) clearance for, its operating room solution hypotension decision assist (HDA)® designed to help better manage hypotension during surgery
Directed Systems selects Advantech as the hardware platform to introduce a new enhanced HDA-OR2 model – a medical grade networked tablet with remote capabilities that will enable access to case review summaries, remote software upgrades and web-based performance metrics HDA will be showcased in the United States for first time at the 75th Anniversary of …